The Global Tricuspid Valve Repair Market is expected to reach a US$2.5 billion market by 2028. Rise in the incidence of tricuspid valve disorders, advancements in medical technology in the field of cardiac interventions, improved awareness of heart valve disorders, a rising geriatric population, growing preference for minimally invasive procedures such as annuloplasty and transcatheter tricuspid valve repair (TTVR), and increase in clinical research and trials activities for developing new tricuspid valve repair technologies are some of the key factors driving the global tricuspid valve repair market. To learn more about the research, fill out a quick inquiry for a sample report.
Download Sample PDF: https://meditechinsights.com/tricuspid-valve-repair-market/request-sample/
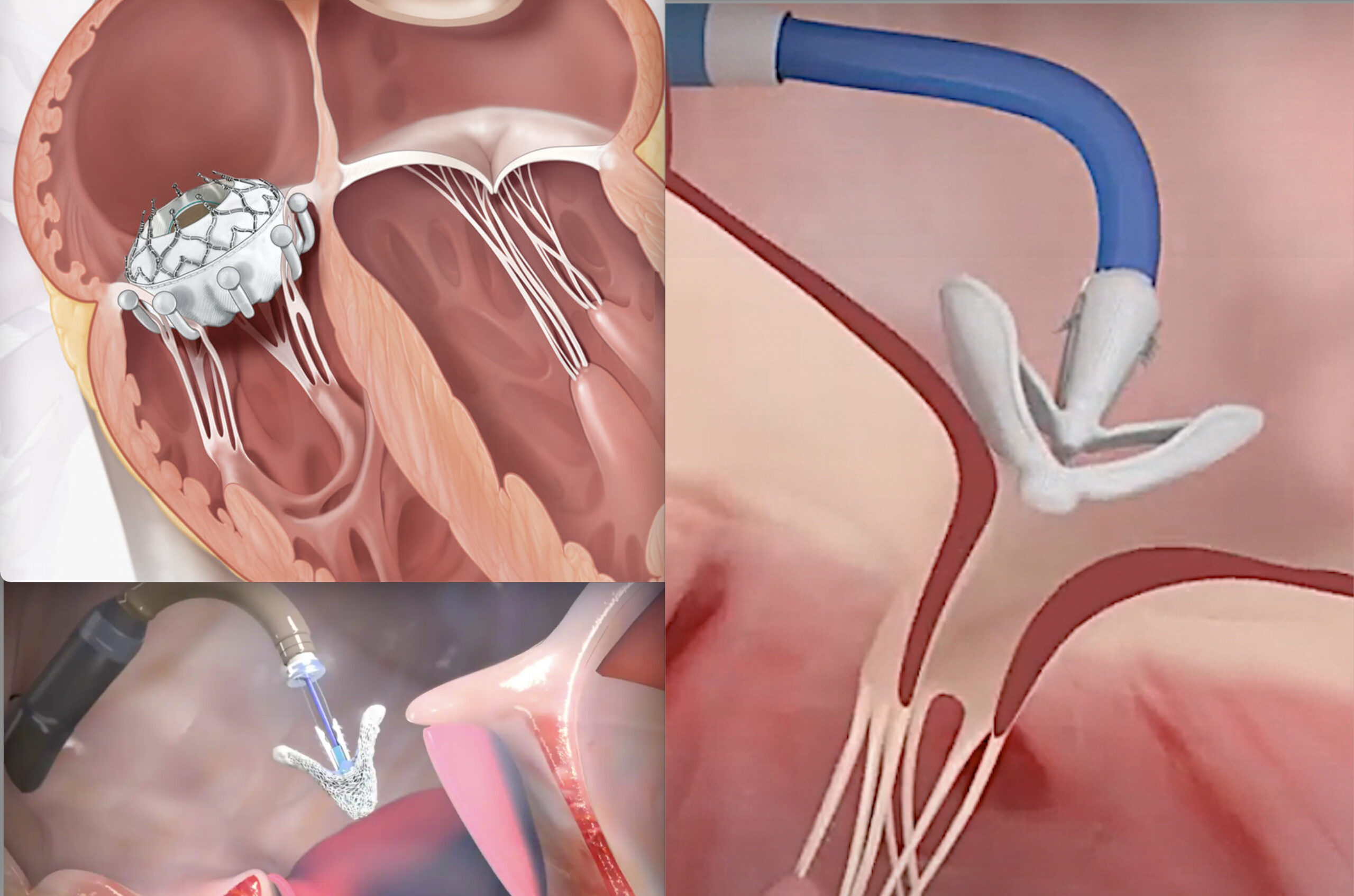
The tricuspid valve is one of four critical valves that regulate blood flow in the heart. Located on the right side, it sits between the right atrium (upper chamber) and the right ventricle (lower chamber), ensuring blood flows in the correct direction. The valve has three flaps, or leaflets, that open and close with each heartbeat, allowing blood to pass from the atrium to the ventricle. However, certain conditions can affect its function, leading to what are known as tricuspid valve diseases. The main types include:
- Tricuspid Regurgitation: The valve doesn’t close tightly enough, causing blood to leak backward into the atrium.
- Tricuspid Stenosis: The valve leaflets become stiff and thickened, limiting blood flow.
Tricuspid valve replacement and repair are key procedures used to treat these conditions. When possible, repair is preferred over replacement with a prosthetic valve. Valve repair typically involves modifying the valve’s tissue or its surrounding structures. A common part of the repair procedure is the implantation of an annuloplasty ring or band. This cloth-covered device is placed around the valve’s annulus (the ring-like structure that holds the valve in place), helping to support the valve and bring its leaflets closer together, potentially reducing leaks. This procedure can improve symptoms and enhance survival rates for patients with tricuspid regurgitation or stenosis.
Download Sample PDF: https://meditechinsights.com/tricuspid-valve-repair-market/request-sample/
Tricuspid Valve Repair Market: Competitive Landscape
The top market players in the global tricuspid valve repair market include Edwards Lifesciences Corp., Abbott, Medtronics, Corcym, Labcor, Valtech Cardio Ltd., Sorin S.p.A., CroiValve, FOLDAX, Cardia Implant LLC, LivaNova PLC, Cyberonics Inc., Boston Scientific Corporation, Micro Interventional Devices Inc., 4Tech Inc., among others.
Rising Demand for Tricuspid Valve Repair Due to Advances in Treating Tricuspid Regurgitation
Tricuspid regurgitation (TR) is the most common reason for tricuspid valve repair. In the U.S., an estimated 1.6 million people suffer from moderate to severe TR, while only a few thousand undergo the procedure each year. This patient group is typically elderly, experiencing symptoms like fatigue, limited exercise tolerance, swollen legs, and abdominal bloating. Medical treatment, including high-dose diuretics, offers limited relief, making surgery the best long-term solution. However, despite the inclusion of tricuspid valve surgery in clinical guidelines, its treatment rate is lower than for other valve diseases, and the mortality rate is higher. Due to significant comorbidities, fewer than 1% of patients are eligible for surgery. Most are left with only medical management to temporarily reduce symptoms.
As a result, there is increasing interest in less invasive procedures compared to traditional open-heart surgery. For example:
- October 2023: Edwards Lifesciences Corporation announced that its EVOQUE tricuspid valve replacement system had received CE Mark approval for the transcatheter treatment of eligible patients with tricuspid regurgitation.
- April 2021: Abbott received CE Mark approval for its next-generation TriClip Transcatheter Tricuspid Valve Repair System, a first-of-its-kind minimally invasive device available in Europe for treating tricuspid regurgitation.
Browse Report: https://meditechinsights.com/tricuspid-valve-repair-market/
About Medi-Tech Insights ;
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde
Associate, Medi-Tech Insights
+32 498 86 80 79